HOW SMALL IS AN ATOM?
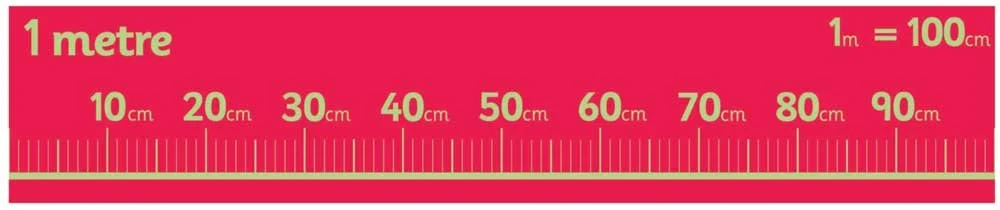
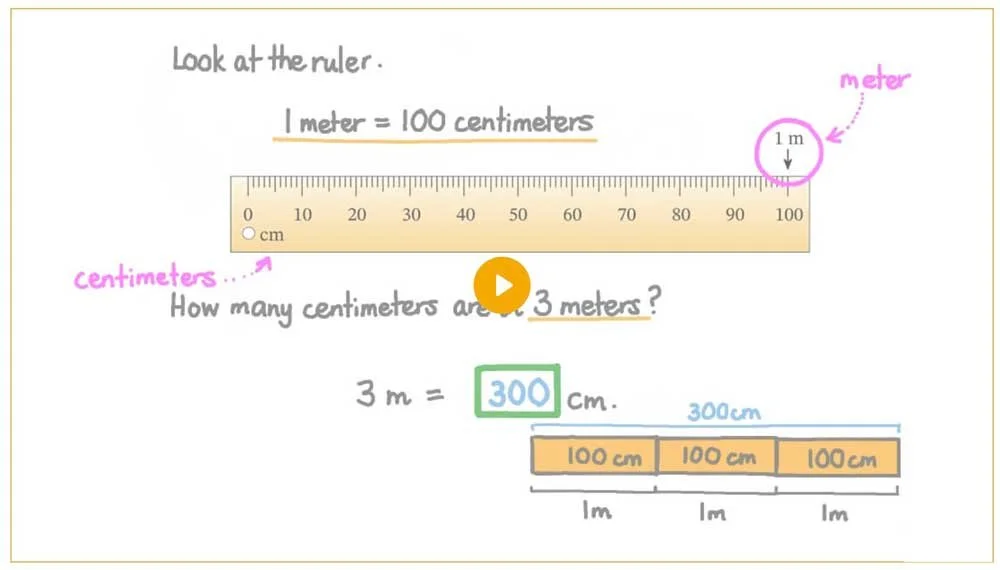
How many centimeters are there in a meter?
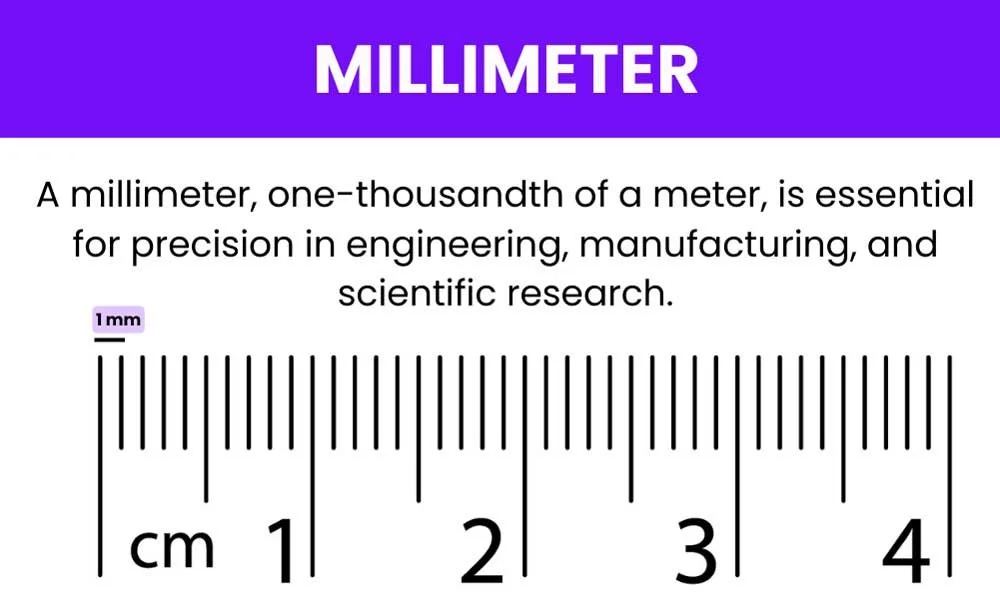
How many millimeters are there in a meter?
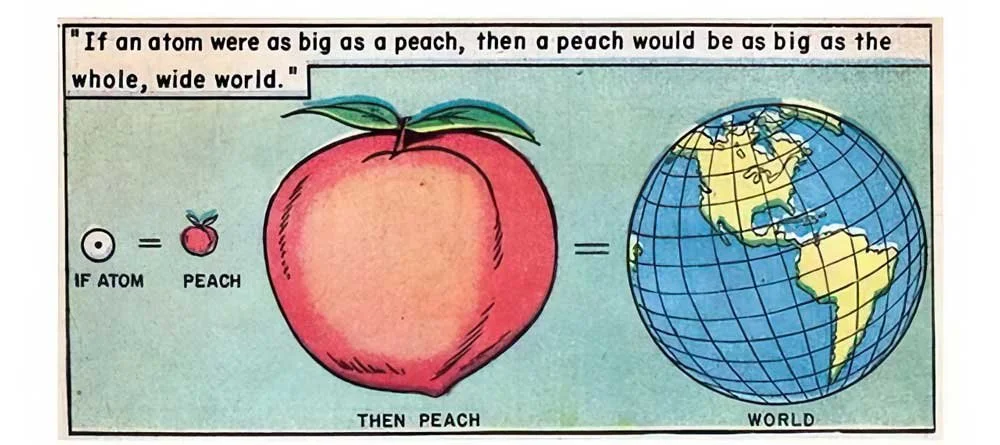
If an atom were as large as a peach, how big would the peach be? (Hint: Look at one of the illustrations below.)
What is the nucleus of an atom made of?
If protons have a positive charge (+), what charge do electrons and neutrons have?
What did the ancient Greeks call the tiny particles that make up everything in the universe?
Do electrons orbit the nucleus like planets orbiting the sun?
What do scientists call the specific shapes where electrons live?
Try to describe the specific shapes where electrons live!
Can you summarize the video and the text? (Hint: There’s a summary at the end of the text!)
• Read •
HOW SMALL IS AN ATOM?
SMALL
What’s the smallest thing you can think of?
Maybe a penny or a button? How about a Cheerio? Its height is about half of a centimeter.
For comparison, there are 100 centimeters in a meter.
Let’s go smaller: a grain of salt. This is about 0.3 millimeters.
There are 1,000 millimeters in a meter.
And even smaller… bacteria are only a few micrometers.
There are 1 million micrometers in a meter. A virus is about 20 to 300 nanometers.
There are 1 billion nanometers in a meter.
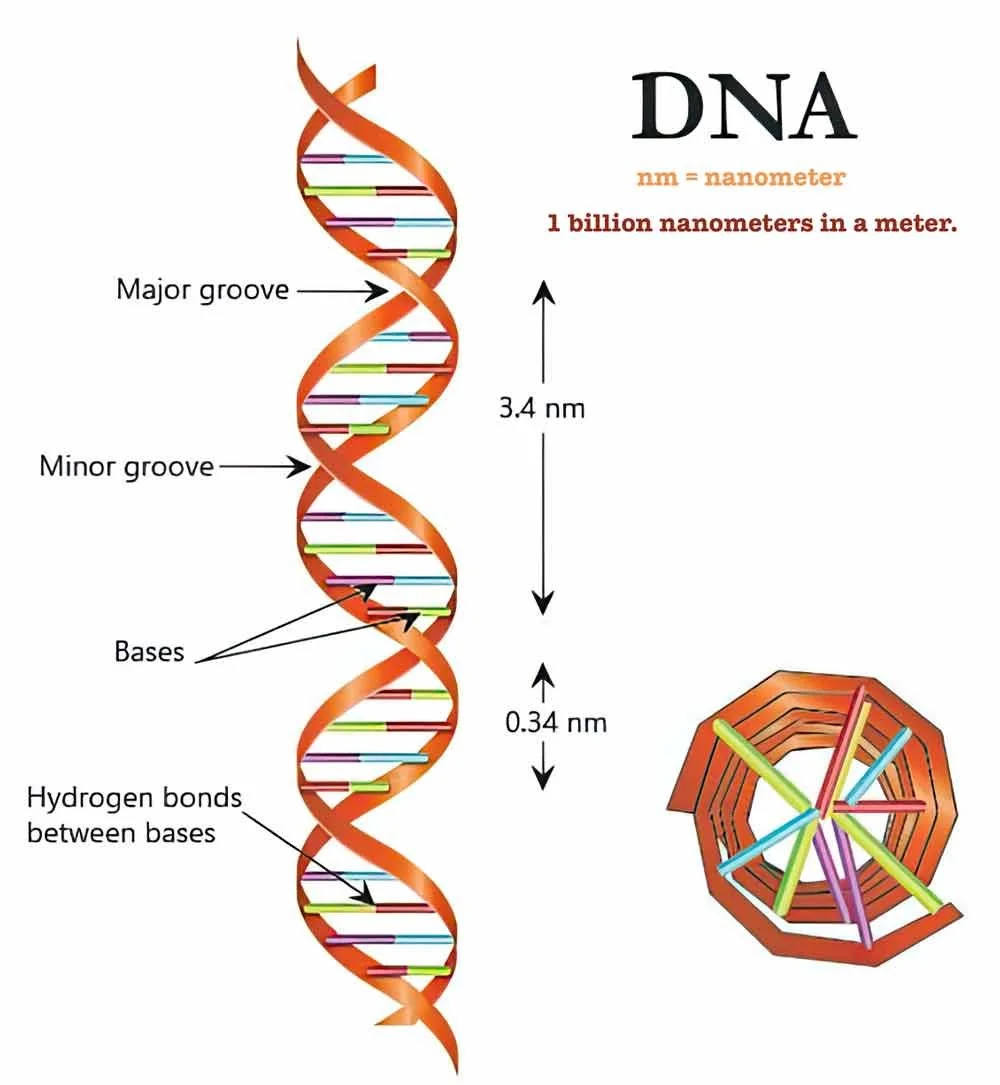
The diameter of DNA is about 2 nanometers.
The size of an atom is only a few angstroms.
There are 10 billion angstroms in 1 meter.
What is an atom?
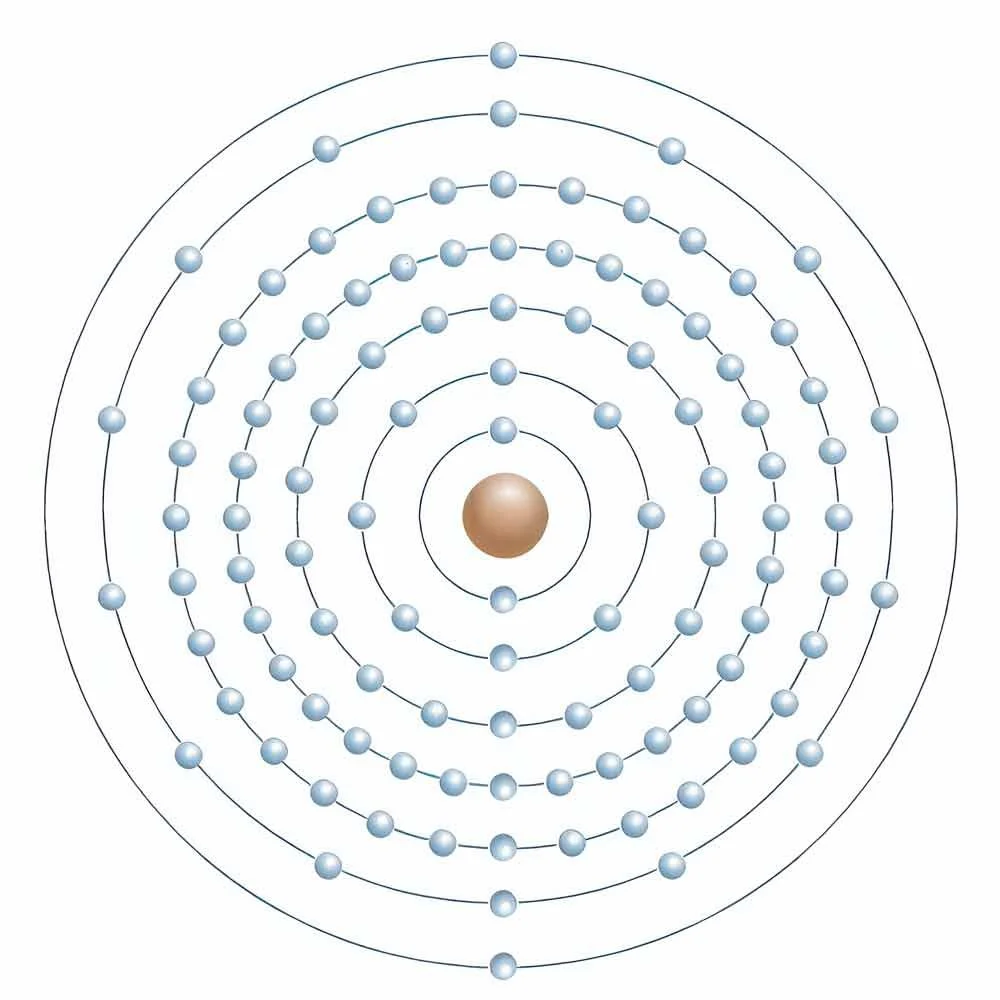
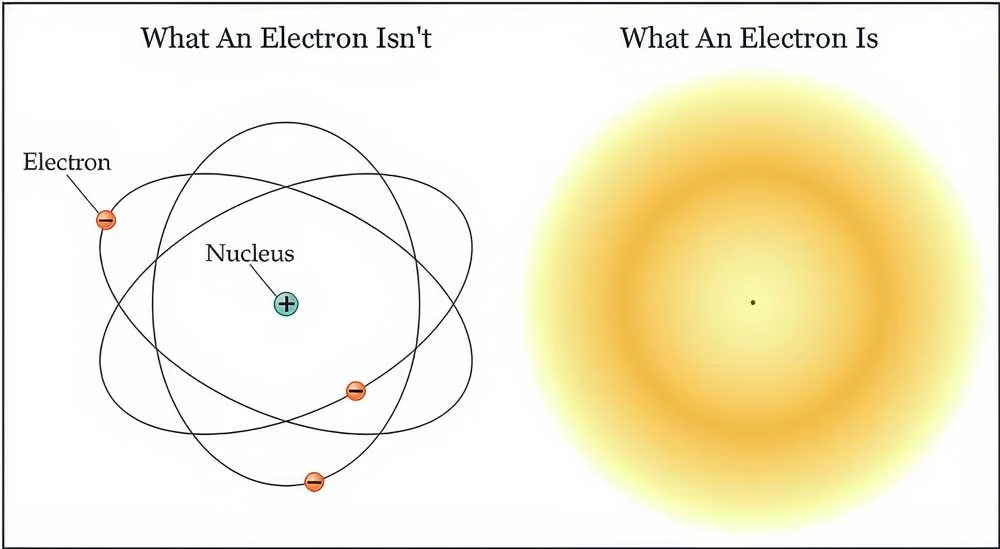
When you think of an atom, you probably picture something like this—on the outside, you’ve got electrons that have a negative charge; in the middle is the nucleus.
The nucleus is made of neutrons, which have no charge, and protons, which have a positive charge.
This model is a good starting point, but there are a few things here that don’t quite agree with modern science.
For one, the size of the nucleus is a lot smaller than this. If I animate it to scale, you wouldn’t even be able to see it. The same thing with these electrons.
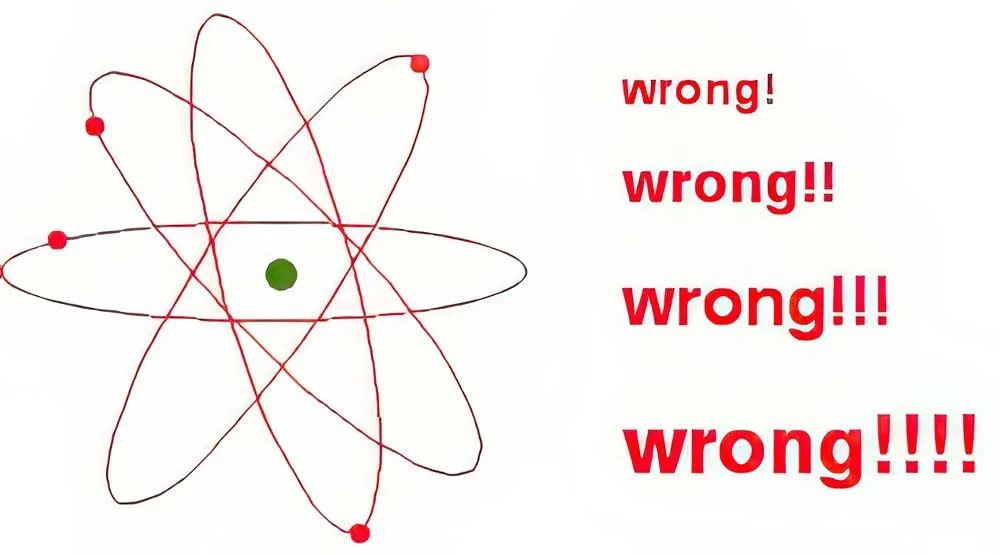
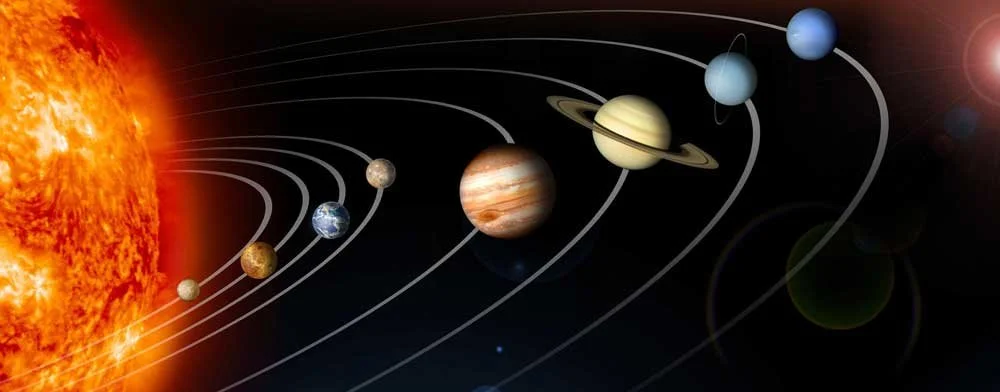
Another thing that’s inaccurate is that electrons orbit the nucleus, just like a planet orbits a star. Unfortunately, this is still taught in many textbooks, but it’s just not correct.
History of the Atom
Let me cover some background first.
Over 2,000 years ago, ancient Greek philosophers had this idea that everything was made of tiny particles. They called these tiny particles atoms.
It wasn’t until the 1800s that we finally started using science to prove that these atoms really exist.
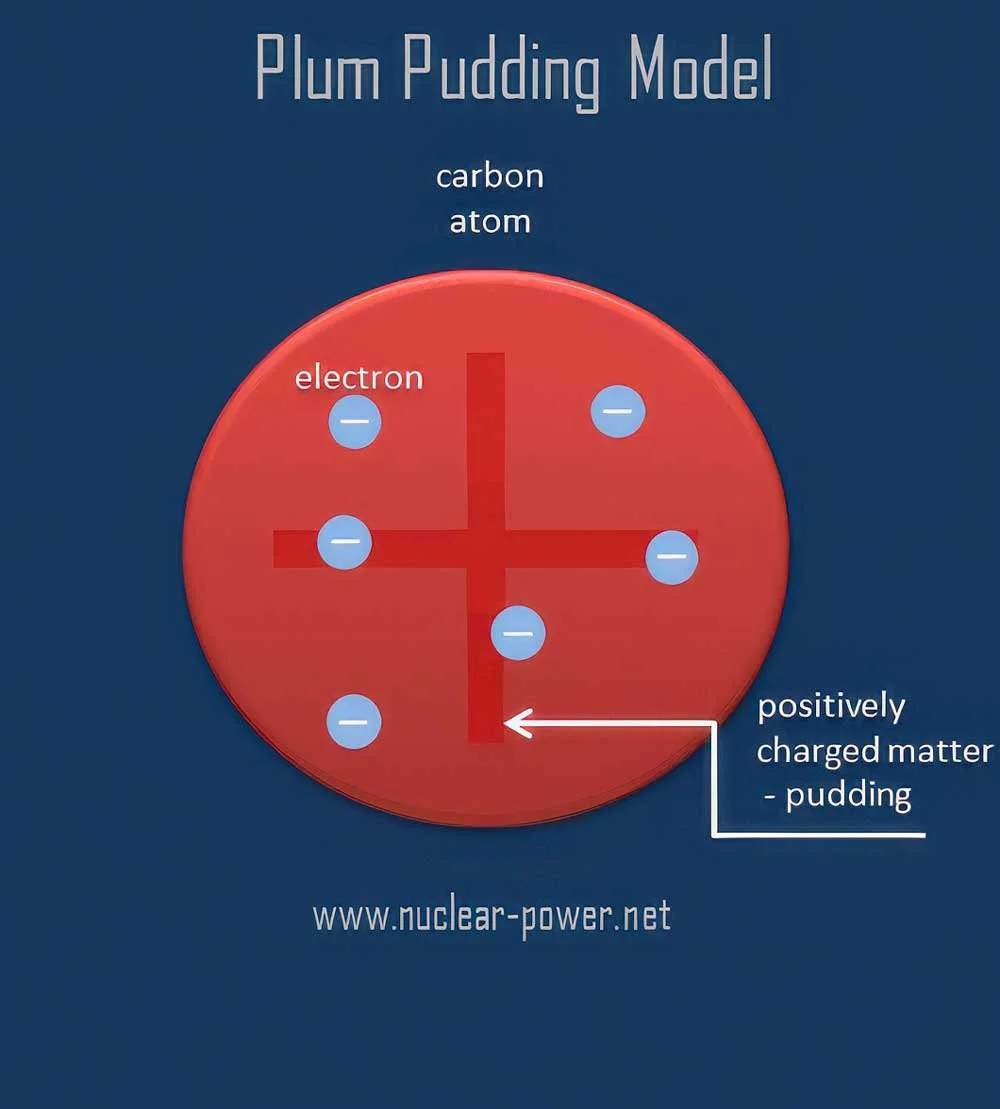
First, we thought atoms looked like this: a positively charged sphere with negatively charged electrons floating around it.
Then we learned that this positively charged sphere was actually a lot smaller. We called this the nucleus. Slowly but surely, we learned that the nucleus is made up of protons and neutrons.
These electrons were tricky.
At first we thought, they have to be doing something, so they probably revolve around the nucleus like this.
Electrons were then discovered to have different energy levels; we call these shells. Shells can only fit a certain amount of electrons; the more electrons, the more shells.
It didn’t take long before we realized that these shells don’t determine how close the electron is to the nucleus.
As it turns out, electrons are a lot more unpredictable.
UNPREDICTABLE ELECTRONS
So if electrons don’t orbit the nucleus, what do they do?
Let’s start with an idea of an orbit.
Here we have the Earth going around the Sun. If the Earth is here today, we can use the laws of physics and gravity to predict exactly where the Earth will be three months from now.
We know both where the Earth is and where it’s going.
Now let’s go to the size of an atom.
With an electron, things are a little different. We can’t know exactly where it is and where it’s going. We can only know one or the other at any given time.
This means it is impossible to really know what the electron is doing.
The best we can do is predict where the electron will be found. This area is most commonly known as the electron cloud.
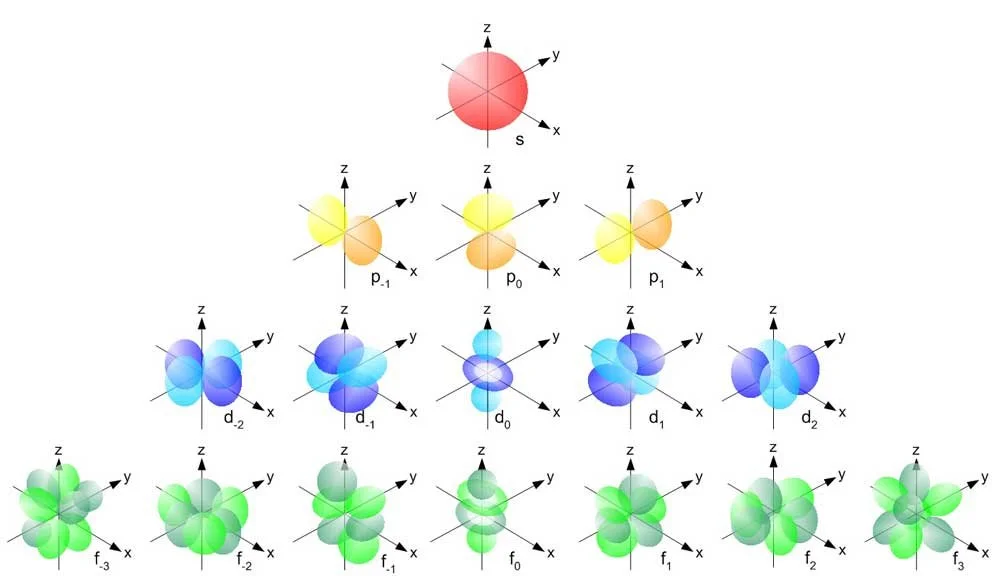
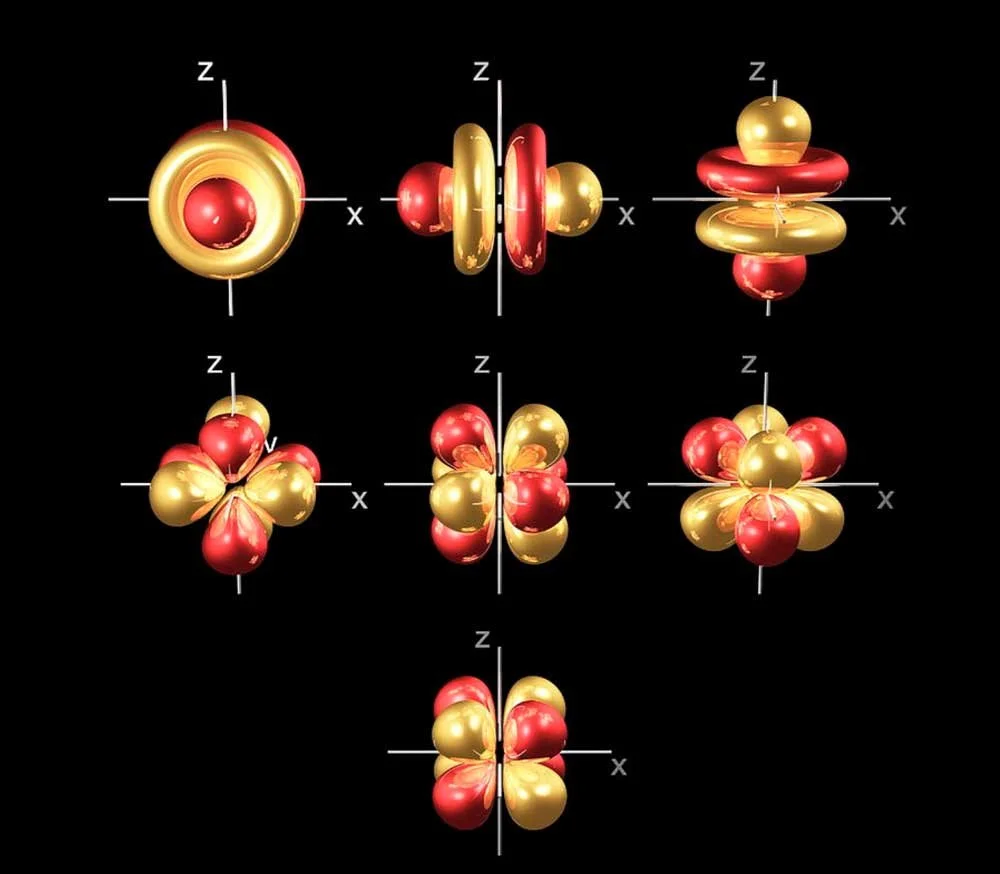
However, if we want to be more specific about where to find electrons, you’ll need to know about orbitals. This is not the same as orbit. Orbitals are specific shapes where electrons live.
• Electron orbitals.
• Electron orbitals.
If you were in a college chemistry class, you’d be studying about how these orbitals fill up as you get more electrons.
But I’d like to keep things simple for this video.
So in short, electrons are uncertain. We can’t know the path that they travel; only that they will be found here, in the electron cloud.
So now you know that popular ways of representing an atom can be misleading.
SUMMARY
Just to recap what we learned:
Everything is made of atoms.
Atoms are incredibly small.
The nucleus is even smaller.
And electrons don’t orbit the nucleus—their paths are unpredictable.
Angstroms (n.) A very small unit of length used to measure atoms and tiny structures; one angstrom is one ten-billionth of a meter.
Atom (n.) The smallest building block of matter; everything around us is made of atoms.
Bacteria (n.) Very small living things made of one cell; some can cause disease, while others are helpful.
Charged (adj.) Having a positive or negative electric force.
Diameter (n.) The distance across a circle or sphere, passing through the center.
Electron (n.) A tiny particle with a negative electric charge that moves around the nucleus of an atom.
Energy levels (n.) Specific places around an atom where electrons are most likely to be found.
Gravity (n.) The natural force that pulls objects toward each other, like how the Earth pulls us down.
Micrometer (n.) A very small unit of length; one million micrometers fit into a meter.
Molecule (n.) A group of two or more atoms joined together.
Nanometer (n.) A unit of length smaller than a micrometer; one billion nanometers fit into a meter.
Negative (adj.) In science, describes an electric charge that is the opposite of positive.
Nucleus (n.) The center part of an atom, made of protons and neutrons.
Orbit (v.) To move around something in a path, like Earth orbits the Sun.
Orbital (n.) The area around an atom’s nucleus where electrons are most likely to be found.
Particle (n.) A very small piece of matter, such as an atom or part of an atom.
Philosopher (n.) A person who thinks deeply about questions like what things are made of or how we should live.
Proton (n.) A tiny particle with a positive charge, found inside the nucleus of an atom.
Shells (n.) Layers around the nucleus of an atom where electrons are arranged.
Unpredictable (adj.) Not able to be known or guessed in advance; surprising or uncertain.
► COMPREHENSION QUESTIONS
— please answer with complete sentences
How many centimeters are there in a meter?
How many millimeters are there in a meter?
If an atom were as large as a peach, how big would the peach be? (Hint: Look at one of the illustrations below.)
What is the nucleus of an atom made of?
If protons have a positive charge (+), what charge do electrons and neutrons have?
What did the ancient Greeks call the tiny particles that make up everything in the universe?
Do electrons orbit the nucleus like planets orbiting the sun?
What do scientists call the specific shapes where electrons live?
Try to describe the specific shapes where electrons live!
Can you summarize the video and the text? (Hint: There’s a summary at the end of the text!)
► From EITHER/OR ► BOTH/AND
► FROM Right/Wrong ► Creative Combination
THESIS — Argue the case that atoms are the smallest thing there is.
ANT-THESIS — Argue the case that maybe atoms are not the smallest thing, maybe atoms can be split up.
SYN-THESIS — Can both perspectives be right?